It's 1 (i) as in the alphabet "I", comes after (h), not the roman numeral for "1"
write a sensible value of the volume.. (within the range) example 50cm3, that could be easily measured and titrated in a lab.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
It's 1 (i) as in the alphabet "I", comes after (h), not the roman numeral for "1"
can u repost any that u found........there r wayy 2 many pages 2 go through...thanksummmm well im havin ALOT of probs in dilutions, i just dont get it?!!!!
omg this helped a lot...finally i got it ...thankyouu@kazi umayer alim
In this experiment we get a precipitate. The prediction that we're putting into test is that the moles of of the precipitated copper hydroxide increases with the increasing conc. of CuSO4.
Thats about it! Hope it helped!
- So keeping our hypothesis in our mind, we should know that the conc. of CuSO4 is the independent variable, which means it is in our control. Hence, you need to prepare a range of concentrations of CuSO4. You're also aware of the fact that the solutions saturates at 1.39 mol/dm3. So the range of concentrations that you prepare must not exceed 1.39 mol/dm^3. So your range is going to be 0-1.39 mol/dm^3. You need to prepare at least five diluted solutions.
- Say you're going to prepare 100 cm^3 of each of the following conc. range: 0.01, 0.05, 1.00 1.10, 1.20 and 1.39. You need to tell the examiners exactly how you're going to prepare them. Since you're given SOLID hydrated CuSO4, you're going to have to dissolve a certain mass in water. So say you want to make the 0.01 mol/dm^3 solution- you need to know how many moles are are to dissolved in a 100 cm^3 solution. So Moles= CV= 0.01*100*10^-3= 0.01 mol. So you need to dissolve 0.01 mol of hydrated CuSo4 in 100 cm^3 of water. To find out the mass= Moles*Mr= 0.01*249.6= 2.50 g. So you dissolve 2.50 g of the salt into 100 cm^3 of water to make a 0.01 mol/dm^3 solution. The rest of the concentrations are to be prepared in the same way- but you don't need to show the working. One calculation for conc. should be enough.
- After you've added the NaOH into the solution a precipitation of Cu(OH)2 will occur. You need to filter this (Or centrifuge it,and then decant).You could leave it out to dry, or add water and propanone.
hello could anyone plz help me with Q1 (h),(i) and Q3 (c)
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s10_qp_51.pdf
thanx could u also help me with this same paper's Q3 (c)question1 (H) Q=mc x change in temp.. where m is the total volume of acid and base ( as we assume 1cm3 as 1g)
this Q is the enthalpy change for the no. of moles of NaOH you used in the experiment. so to find enthalpy change neutralization, i,e part (i) find the enthalpy change for 1 mole using the above expression ( of part h)
pls can someone show or explain how to do part 1. (e) and (d) of this question ?
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w12_qp_52.pdf
thanxx alotFor Q1 i, see my post above.
For 3 (c)
You have take any coordinate from the graph (except for the anomalous point). Say you took the coordinate (1.50, 2.45)
You have to calculate the no. of moles of both the Mg and MgO
So for Mg= 1.50/24.3= 0.06 mol
For MgO= 2.45/40.3= 0.06 mol
ratio 1:1
If we see the equation for the reaction of Mg with O2:
Mg + 1/2 O2 ----> MgO
You can see that the Mg and the MgO are in a 1:1 ratio. And we calculated the moles of a point, which was also 1:1, hence it corresponds to the formula of MgO
please could somebody help me with Q. 1 d) i) on how to find the concentration of H2O2 when volumes of H2O2 and H2O are chosen.
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w10_qp_53.pdf
Thank youu!
let the total volume.. 50cm3
for 2.00mol/dm3.. you need 50 cm3 of aq hydrogen per oxide...
for 1.8mol/dm3 .. you need x cm3 of aq hydrogen per oxide..
2.00 ------> 50
1.8 -------> X
X x 2 = 50 x 1.8
find the value of X>. you will get the volume of H2O2 for 1.8mol/dm3 concentration
subract 50-X to get the volume of water
thankssss!For the diagram:
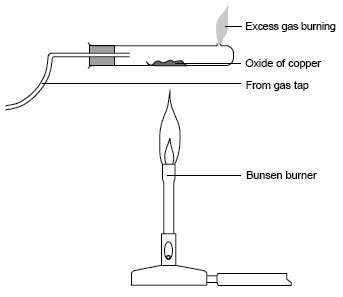
Replace the Copper with Lead though!
This is how you will heat the Lead oxides, with the excess hydrogen burning at the end of the tube. And instead of pipe to the gas tap, you should attach a syringe. And in another diagram on the same page, you should draw a a test tube, with a rubber bung, attached to the same syringe. In the contents of the tube, mention a gorup II metal (For eg: Mg) + a suitable acid, like HCl. This will liberate H2 gas into the syringe, which you will then attach to the apparatus shown above.
In part i, you're asked to derive an expression for enthalpy change of neutralization. This energy is going to be given off by the acid and the base reaction. You already know that the energy given by the reacition is (vol/mass NaOH + vol/mass H2SO4) × 4.3 × ∆T.
Look at the reaction. the ratio between NaOH and water is 1:1 (REMEMBER: The enthalpy change of neutralization is when 1 mole of water forms). So for the energy released by 1 mole of NaOH, 1 mole of H2O is produced. So if 'n' moles of NaOH were present, 'n' moles of water would form. Now, since enthalpy change of neutralization is the ENERGY when ONE MOLE of water is formed, we can derive our expression now. Energy was (vol/mass NaOH + vol/mass H2SO4) × 4.3 × ∆T per 'n' number of moles.
So the expression will be [(vol/mass NaOH + vol/mass H2SO4) × 4.3 × ∆T]/n
And this this is an EXOTHERMIC reaction, it will be -ve.
Sorry bro i meant c not d! please if u can helpApparatus is hot, so handle with heat proof gloves.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now