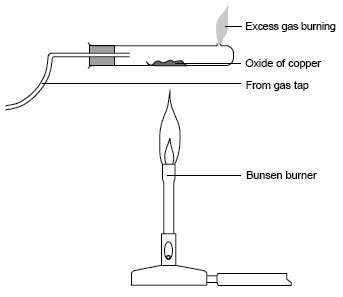
anybdy?!
cud sme1 plz tell me d apparatus whch z to be drawn for ques 1d in oct-nov year 12 variant 2 ?!
here z d link ----> http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_52.pdf
cud sme1 plz tell me d apparatus whch z to be drawn for ques 1d in oct-nov year 12 variant 2 ?!
here z d link ----> http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_52.pdf