- Messages
- 1,983
- Reaction score
- 3,044
- Points
- 273
yesIS THIS OK?
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
yesIS THIS OK?
if you see the the fig 5.2 you will realize the total wave produce are 3/2
use the formula E=1/2*k*(x^2)What about the second part (2.)
new wave will be straight line.http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Physics (9702)/9702_s13_qp_22.pdf
guys how are we suppose to do q 5part b iii i dont get how we r suppose to draw the new wave
I just did fag -_-http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Physics (9702)/9702_w09_qp_22.pdf
Can anyone PLEASE explain Q4 c ii) full
sorry dude if I am bothering you too muchuse the formula E=1/2*k*(x^2)
you do know the formula for elastic potential energy, right?sorry dude if I am bothering you too much
can you please explain it
Change in U = mgh = 2.8N * 0.015 m = 0.057Jhttp://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Physics (9702)/9702_w09_qp_22.pdf
Can anyone PLEASE explain Q4 c ii) full

Idon't get how does this represent diffractionHere you need to draw labelled diagram of source of light, apparatus to diffract/interfere and how to observe it.
To observe the diffraction the light must be perpendicular to the slit and the screen must be long distance from slit
to observe. we will decrease slit separation(in (ii) ) and slit width in (i) part.
(i) when we will decrease it, the the spot will start to spread into geometric shadows. along the bright big spot a few fringes will also be observed but they will be dim.
(ii) the fringe seperation will increase. alternate bright and dark fringes will be produced.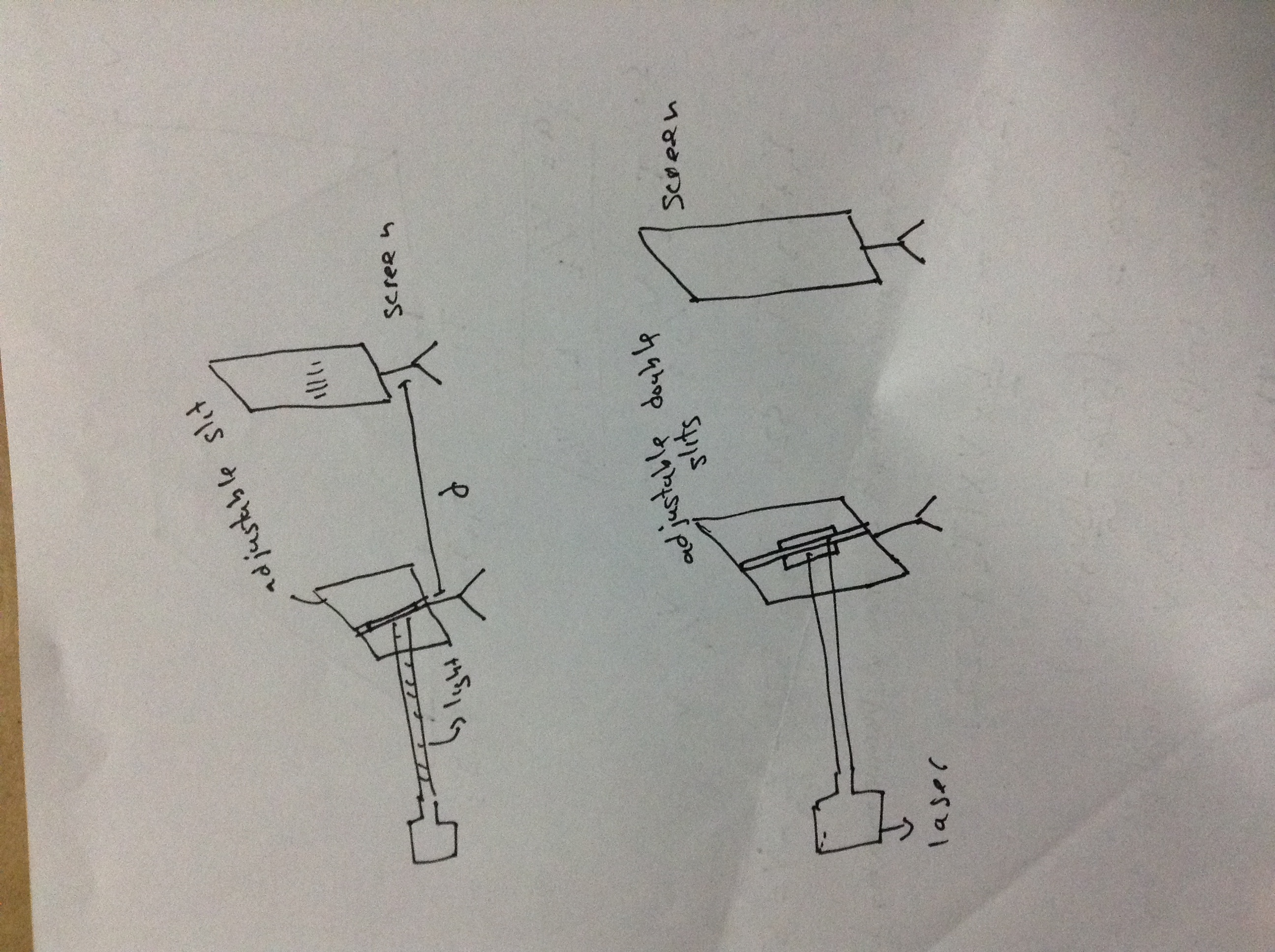
correct. but i drew it to make it easy for you to understand. i always use cosine rule or Pythagoras theorem unless examiner himself says to draw scale drawing.But the question didn't ask for drawing such diagrams ! I did this way and got the answer ! but I'm not sure did this answer 1 e (i) or not ! please someone correct me If I'm wrong !
Suchal Riaz
the laser is normal to a slit and that difracted light goes to screen. right click that image and open it in new tab because the image is rotated in this website.I
Idon't get how does this represent diffraction
avoid using abusive words such as fag again. if you solved and he didn't get then it doesn't mean you will get rude to him. and the way you explained no one would get it. read the terms and rules of this forum: https://www.xtremepapers.com/community/help/termsI just did fag -_-
please explain him that question in which i tagged you then i will answer your doubt.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now