- Messages
- 500
- Reaction score
- 425
- Points
- 73
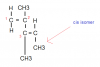
View attachment 63883 View attachment 63884 View attachment 63885 View attachment 63886 View attachment 63887 I need help with these.
Can anyone explain why 30 isn’t C. According to what I’ve learnt about sigma bonds, 30 should be C.
Q: 3