- Messages
- 63
- Reaction score
- 31
- Points
- 18
I cant understand the mass spectrometer, my teacher didn't teach us anything about it.. Help!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
it is! :OAnd check this out :
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s03_qp_2.pdf
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s03_ms_1 2 3 4 5 6.pdf
isnt the MP graph for question 4A wrong? Since there is a slight increase from P to S in melting point?
is it needed for AS?I cant understand the mass spectrometer, my teacher didn't teach us anything about it.. Help!
Which book are you using.. my book explains it pretty well.. i'm assuming you have the same oneI cant understand the mass spectrometer, my teacher didn't teach us anything about it.. Help!
Nice... thanks.. it explained everythingBon enthalpies? You're going to have to elaborate on that
As far as bond breaking and making is concerned, look at the following reaction:
CO + 1/2O2 -> CO2.
Simple enough right? Now all you have to remember is before reacting all atoms must go into their atomic gaseous state. Thus here, the O2 molecule will be broken into 2 O ATOMS. This is bond BREAKING. Then, the O atom will react with the CO molecule to make a CO2 molecule. During this, the C bonds with the extra O atom. And this is bond MAKING. Bond breaking is always ENDOTHERMIC because it always requires energy. Thus, Bond making is automatically exothermic.
Think of bond making as a bank transaction. When you're MAKING a bond you're putting in money. Now theres less money [Energy] in your pocket [surroundings], but more money [Energy] in your bank account [between bonded atoms]. Likewise when you make a withdrawal or BREAK a bond, there is less money in your bank account, but more in your pocket.
Mass spectrometry is only in A2.. what are you guys talking about?Mass spectrometer for AS is simple enough. You ionize/vaporize a sample, throw electrons at it until it loses an electron going into the +1 state, and pass it through electric field [to streamline it] and magnetic field [to make it acquire a circular pathway]. Then you change the magnetic field to make it hit your detector, giving you a mass/charge ratio against percentage abundance of said peak.
But you still manage to embarrass us A2 candidates by answering our (at least mine) stupid basic questionsLooks like almost everyone here is A2
I'm still AS.
Yeah it seemed like that to me to..... nice to find a fellow AS here *hi5Looks like almost everyone here is A2
I'm still AS.
You got it wrong there, all alkali are bases bot not all bases are alkaline. This is because alkali's are bases which are dissolved in water, like NaOH(aq) for example.
Reactivity mainly depends on the effective nuclear charge, atomic radius, and molar mass (not really sure about molar mass lol).
Check it out.. daredevil likes my depressing reply to PandaYeah it seemed like that to me to..... nice to find a fellow AS here *hi5
whoa awesome you knew so much stuff i thought you'd be in A2 .. if not higher upLooks like almost everyone here is A2
I'm still AS.
I'd suggest to draw the cycle every time for questions like this.Hey can anyone step by step explain Q1 (c) for MJ12-41 ??
I always get SIMPLE lattice energy or enthalpy change questions in general wrong :/
I'd be forever greatfuk(
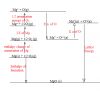
Whats your question?i guess i'll just google my question?
yep thats right^
btw panda... i need a bit of help
in organic chemistry we have oxidising agents KMnO4 and K2Cr2O7
and some reactions state using only one.. is there a difference? similarly LiAlH4 and NaBH4..
yep thats right^
btw panda... i need a bit of help
in organic chemistry we have oxidising agents KMnO4 and K2Cr2O7
and some reactions state using only one.. is there a difference? similarly LiAlH4 and NaBH4..
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now