-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 187
- Reaction score
- 191
- Points
- 53
View attachment 52033
how is the ans B?
For 1, bromoethane from ethanol is a reaction that requires heating an alcohol under reflux, and then distilling off the halogenoalkane. The alcohol being ethanol here and the halogenoalkane formed being bromotethane.
The very fact that the halogenoalkane has to be distilled can confirm the necessity of having such a set up, since the halogenoalkane can be collected as a distillate in the flask attached to the end of the liebig condenser.
For 2, ethanal forming from ethanol is due to the oxidation of ethanol. This does require for the reaction mixture to be warmed before the oxidation takes place, and in the end you are also required to collect the aldehyde as a distillate.
For 3, 1,2-dibromoethane forming from bromine and ethene is done by bubbling an alkene through a solution of a halogen on the form X2.
So for 1.2-dibromoehane, it would simply have to be bubbled through a solution of bromine at room temperature ( aka no heating required ).
From all of the above options, the only two that require heating and distillation to take place are 1 and 2. Which is option B.
Hope that helped
- Messages
- 187
- Reaction score
- 191
- Points
- 53
View attachment 52035
why isnt it C?
Both phosphorus and sulfur are non-metallic elements that form acidic oxides, and eventually strong acids in water.
As for their electronic configurations,
Phosphorus is 1s2,2s2,2p6,3s2,3p3 ------> each of the 3 electrons are split into 3px, 3py and 3pz. Hence there are no "paired" electrons.
For Sulfur however, the electronic configuration is 1s2,2s2,2p6,3s2,3p4 ---------> There are 4 electrons, First, 3 electrons fill 3px, 3py and 3pz, followed by the 4th electron going into 3px. This means that sulfur does have " paired " 3p electrons.
So for Phosphorus, both statements are correct and for Sulfur, statement 2 is false. Your final answer will be A.
- Messages
- 187
- Reaction score
- 191
- Points
- 53
N2 + 3H2 <---> 2NH3
Habers process involves an exothermic reaction. An exothermic reaction favours a lower temperature, and would produce yield at a faster rate and overall increase the total yield.
Secondly you need to look at the x-axis, which is pressure. A higher pressure, would favour the side with a lower number of moles. There's 4 moles on the left compared to 2 on the right. This results in the reaction favouring the production of ammonia, since there are fewer moles on the right.
With this in mind, you need to have a graph with a positive gradient since that would mean the yield increases with pressure, as well as a graph where the lower temperature has the higher overall yield.
That graph is A.
Hope that helped
- Messages
- 735
- Reaction score
- 2,652
- Points
- 253
21/M/J/12
Question 5b(iii) Can some one please explain?
and in same Question c(ii) part they said use your information from b(i),b(ii) & c(i)..........
How can we use the part b(ii) answer to help us in this question????
Question 5b(iii) Can some one please explain?
and in same Question c(ii) part they said use your information from b(i),b(ii) & c(i)..........
How can we use the part b(ii) answer to help us in this question????
- Messages
- 187
- Reaction score
- 191
- Points
- 53
21/M/J/12
Question 5b(iii) Can some one please explain?
and in same Question c(ii) part they said use your information from b(i),b(ii) & c(i)..........
How can we use the part b(ii) answer to help us in this question????
1 molecule of X produces 2 hydrogen atoms. The hydrogen atoms come off of the -OH group as H+, leaving behind -ONa.
The reaction is, X + Na ---------> XNa + H2
The -OH group in X, has H replaced by Na. This gives 1 hydrogen atom.
In the products we have two hydrogen atoms, so there has to be another -OH group present.
------------------------------
I'm assuming when they say use your answer to b) ii) they're referring to the fact that 2 hydrogen atoms are formed. So X must have 2 hydrogen atoms.
However I find there's a much easier way to deducing the structure of X and it can probably save you a lot of time as well,
They tell you at the start the Mr of X is 90. The empirical formula of X being CH2O.
C = 12 g, H = 1 g, O = 16 g
Using the ratio of 1 C: 2 H : 1 O you get 12+2+16 = 30 g
You can use this to find out how much C, H and O contribute to 90 g.
90/30 * 1:2:1, since that is the ratio of C:H:O in the empirical formula.
This gives you C3H6O3 which is the molecular formula of X.
A chain of 3 carbons, CHO must be at the end of the chain. The 2 OH groups can be on the same carbon, or one on each.
You can then attach the remaining C and H atoms depending on where a bond can be made.
Route 1 ------> CH(OH)2CH2CHO
Route 2 ---------> CH2(OH)CH(OH)CHO
Route 3 -----------------> CH3C(OH)2CHO
Hope that helps
thnk u HELPED A LOTFor 1, bromoethane from ethanol is a reaction that requires heating an alcohol under reflux, and then distilling off the halogenoalkane. The alcohol being ethanol here and the halogenoalkane formed being bromotethane.
The very fact that the halogenoalkane has to be distilled can confirm the necessity of having such a set up, since the halogenoalkane can be collected as a distillate in the flask attached to the end of the liebig condenser.
For 2, ethanal forming from ethanol is due to the oxidation of ethanol. This does require for the reaction mixture to be warmed before the oxidation takes place, and in the end you are also required to collect the aldehyde as a distillate.
For 3, 1,2-dibromoethane forming from bromine and ethene is done by bubbling an alkene through a solution of a halogen on the form X2.
So for 1.2-dibromoehane, it would simply have to be bubbled through a solution of bromine at room temperature ( aka no heating required ).
From all of the above options, the only two that require heating and distillation to take place are 1 and 2. Which is option B.
Hope that helped
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
is it true that warm conc kmno4 doesn't react with alcohol at all?
- Messages
- 735
- Reaction score
- 2,652
- Points
- 253
Thanks a lot.Great Explanation1 molecule of X produces 2 hydrogen atoms. The hydrogen atoms come off of the -OH group as H+, leaving behind -ONa.
The reaction is, X + Na ---------> XNa + H2
The -OH group in X, has H replaced by Na. This gives 1 hydrogen atom.
In the products we have two hydrogen atoms, so there has to be another -OH group present.
------------------------------
I'm assuming when they say use your answer to b) ii) they're referring to the fact that 2 hydrogen atoms are formed. So X must have 2 hydrogen atoms.
However I find there's a much easier way to deducing the structure of X and it can probably save you a lot of time as well,
They tell you at the start the Mr of X is 90. The empirical formula of X being CH2O.
C = 12 g, H = 1 g, O = 16 g
Using the ratio of 1 C: 2 H : 1 O you get 12+2+16 = 30 g
You can use this to find out how much C, H and O contribute to 90 g.
90/30 * 1:2:1, since that is the ratio of C:H:O in the empirical formula.
This gives you C3H6O3 which is the molecular formula of X.
A chain of 3 carbons, CHO must be at the end of the chain. The 2 OH groups can be on the same carbon, or one on each.
You can then attach the remaining C and H atoms depending on where a bond can be made.
Route 1 ------> CH(OH)2CH2CHO
Route 2 ---------> CH2(OH)CH(OH)CHO
Route 3 -----------------> CH3C(OH)2CHO
Hope that helps
- Messages
- 735
- Reaction score
- 2,652
- Points
- 253
- Messages
- 187
- Reaction score
- 191
- Points
- 53
Question 1:
2 C14H30 + 43 O2 ----------> 28 CO2 + 30 H2O
1 tonne = 1000 kg
x tonnes = 10.8kg
x = 0.0108 tonnes for 1 km
For 8195 km = 8195 * 0.0108 = 88.506 tonnes
Molar mass of C14H30 = 198g ---> 0.198 kg ------->1.98 x 10^-4 tonnes
88.506 / 1.98 x 10^-4 = 447,000 moles of C14H30 used for 8195 km.
2 moles of C14H30 ----> 28 moles of CO2.
447,000 moles of C14H30 ------> x moles of CO2.
x = 6,258,000 moles of CO2 formed.
Mass of CO2 formed = moles of CO2 * molar mass of CO2 in tonnes
Molar mass of CO2 = 44g = 0.044 kg = 4.4 x 10^-5 tonnes
Mass of CO2 formed = (4.4 x 10^-5) * 6,258,000 =275.352 tonnes of CO2.
Which is basically 275 tonnes.
-------------------------------
reaction 1 is W ----> Z. First find Z,
This is Ca(OH)2 + H2SO4 ------> CaSO4 + 2H2O, Since Z is (s), it should be CaSO4.
W is Ca(NO3)2. Your goal is to go from Ca(NO3)2 -------> CaSO4.
So you end up with something like this,
Ca(NO3)2 + XSO4 ------> CaSO4 + X(NO3)2
or
Ca(NO3)2 + X2SO4 -------> CaSO4 + XNO3
The only two possible options for X in this case, are elements from group 1 and group 2.
Hope that helped!
- Messages
- 187
- Reaction score
- 191
- Points
- 53
is it true that warm conc kmno4 doesn't react with alcohol at all?
Nope. It does react with alcohols, just not under warm conditions. They don't recommend using it because say for instance you want to prepare an aldehyde from a primary alcohol, KMNO4 will end up over oxidizing the alcohol all the way until it becomes a carboxylic acid.
So yes it does react with alcohols, just not under the same conditions as the alkenes.
- Messages
- 735
- Reaction score
- 2,652
- Points
- 253
Thanks for the help Buddy.........Question 1:
2 C14H30 + 43 O2 ----------> 28 CO2 + 30 H2O
1 tonne = 1000 kg
x tonnes = 10.8kg
x = 0.0108 tonnes for 1 km
For 8195 km = 8195 * 0.0108 = 88.506 tonnes
Molar mass of C14H30 = 198g ---> 0.198 kg ------->1.98 x 10^-4 tonnes
88.506 / 1.98 x 10^-4 = 447,000 moles of C14H30 used for 8195 km.
2 moles of C14H30 ----> 28 moles of CO2.
447,000 moles of C14H30 ------> x moles of CO2.
x = 6,258,000 moles of CO2 formed.
Mass of CO2 formed = moles of CO2 * molar mass of CO2 in tonnes
Molar mass of CO2 = 44g = 0.044 kg = 4.4 x 10^-5 tonnes
Mass of CO2 formed = (4.4 x 10^-5) * 6,258,000 =275.352 tonnes of CO2.
Which is basically 275 tonnes.
-------------------------------
reaction 1 is W ----> Z. First find Z,
This is Ca(OH)2 + H2SO4 ------> CaSO4 + 2H2O, Since Z is (s), it should be CaSO4.
W is Ca(NO3)2. Your goal is to go from Ca(NO3)2 -------> CaSO4.
So you end up with something like this,
Ca(NO3)2 + XSO4 ------> CaSO4 + X(NO3)2
or
Ca(NO3)2 + X2SO4 -------> CaSO4 + XNO3
The only two possible options for X in this case, are elements from group 1 and group 2.
Hope that helped!
- Messages
- 735
- Reaction score
- 2,652
- Points
- 253
21/O/N/09
Question 5 (d) help??????????
I usually face trouble when it comes to these types of questions any tips on how to do them????
Question 5 (d) help??????????
I usually face trouble when it comes to these types of questions any tips on how to do them????
- Messages
- 138
- Reaction score
- 31
- Points
- 38
Y is an organic compund. Y gives ppt aqueous silver nitrate. All of this ppt dissolves when concentrated ammonia is added. What is possible identity of Y ?
1- bromopropane
2-chlororethane
3-iodo-2-methylpropane
1- bromopropane
2-chlororethane
3-iodo-2-methylpropane
- Messages
- 138
- Reaction score
- 31
- Points
- 38
Which isomer of C4 H10 O forms three alkenes on dehydration ?
A . butan-1-ol
B . butan-2-ol
C. 2-methylpropan-1-ol
D 2-methylpropan-2-ol
A . butan-1-ol
B . butan-2-ol
C. 2-methylpropan-1-ol
D 2-methylpropan-2-ol
- Messages
- 187
- Reaction score
- 191
- Points
- 53
21/O/N/09
Question 5 (d) help??????????
I usually face trouble when it comes to these types of questions any tips on how to do them????
Errr... This question is quite tricky to explain partially because of the fact that the question is based off of the syllabus back in 2009.
Anyways, the process of forming the cyclic compound from J, is known as cyclic esterification. Cyclic esters are actually called lactones.
The process is as follows, if you have a hydroxy-carboxylic acid ( Hydroxypropanoic acid in this question ), you can have it undergo an "intra"molecular reaction.
Normally during any esterification process, you want a carboxylic acid, alcohol and some heat. However in cyclic esterification, you do not need the presence of an alcohol. You can make do with simply having a hydroxy carboxylic acid and some heat.
That is where the term " intra " comes into play, they have the hydroxycarboxylic acid react within itself to form an ester that has the ability to form a cyclic compound.
There's some advanced theory that involves the inclusion of a dynamic equilibrium, hence the requirement of a catalyst is necessary, more specifically an acid catalyst.
In this question, you have your hydroxycarboxylic acid ( hydroxypropanoic acid ) and an acid catalyst ( Conc. sulfuric acid. ). You're also told that the molecular formula is C6H8O4.
At the start of the question, it mentions that J has 3 carbons. If the molecular formula of L has 6 carbons, that means two molecules of J have been used, and so 2 ester groups will form.
With the two Ester groups, you can have them attach in the shape of a ring ( forming a cyclic compound ), both of them having the formula RCOO-, the diagram in the marking scheme is a good example of how they attach.
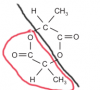 The red part is one ester group. You can tell since R is C2H4 and then you have COO.
The red part is one ester group. You can tell since R is C2H4 and then you have COO.I would just recommend knowing what is required to make a cyclic ester, and the basic ester group notation. The rest is far too complicated for AS level, since lactones are technically part of the A2 syllabus.
Hope that helped
- Messages
- 187
- Reaction score
- 191
- Points
- 53
Y is an organic compund. Y gives ppt aqueous silver nitrate. All of this ppt dissolves when concentrated ammonia is added. What is possible identity of Y ?
1- bromopropane
2-chlororethane
3-iodo-2-methylpropane
When the question states that Y gives a ppt "with" aqueous silver nitrate, you should immediately be able to remember that this is a test for halide ions. Which are the group 7 elements consisting of Cl, Br and I.
The second part of the question is also important, since it can help us eliminate an option.
All 3 compounds, give a ppt with aqueous silver nitrate.
Cl- is soluble in aqueous ammonia.
Br- is slightly soluble in "dilute" ammonia, but soluble in "concentrated" ammonia.
I- is insoluble in both, dilute and concentrated.
Since Iodine disagrees with statement two, which is that it dissolves in conc. ammonia when it clearly does not, we can eliminate option 3.
Both option 1 and 2 have halide ions that are soluble in concentrated ammonia, so they are correct.
Your answer should be B, since that states that both option 1 and 2 are correct.
Hope that helped!


