- Messages
- 316
- Reaction score
- 465
- Points
- 28
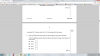
from where have you got 25.9 ?e) if 25.9 --> 0.0025 moles
1000 (1dm^3)---> x
25.9x= 2.5
x= 0.0965 moles
Then the formula no. of moles= mass in g/mr
0.0965= 8.50/x
x=88 g
Now seee this CnH2n+1COOH
Total mass= 88g.
COOH= 45g
CnH2n+1= 88-45
= 43g
C3H2(3)+1= 43g.
Therefore the formula is= C3H7COOH.
Need help with the next part?