- Messages
- 537
- Reaction score
- 105
- Points
- 38
W.S!
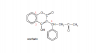
Kp is simply the equilibrium constant in terms of partial pressures. Since gases can occupy any container, there volume is indefinite. The Kp provides a better measure of the equilibrium constant in reactions involving gases as conc. = moles/volume, which is difficult to determine.
To find partial pressure, use: p = n/N x P, where
n: no. of moles of the particular gas (whole partial pressure is being found)
N: total no. of moles (of all gases)
P: total pressure (of all gases).
Once you have the partial pressures, Kp is found in exactly the same way as Kc. Just use the partial pressures where you'd use concentrations.
Thanks!!