- Messages
- 3,063
- Reaction score
- 1,831
- Points
- 173
but there r 2moles of Na in the frst equation?1 moll of NaN3 produces 1.5N2 according 2 equation....but it also produces 1 mol Na which gives 0.1mol of N2 so in total it is 1.6mol of N2
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
but there r 2moles of Na in the frst equation?1 moll of NaN3 produces 1.5N2 according 2 equation....but it also produces 1 mol Na which gives 0.1mol of N2 so in total it is 1.6mol of N2
2mol of Na from 2mol NaN3 thus 1mol Na from 1 Mol NaN3......but there r 2moles of Na in the frst equation?
oh yea sory2mol of Na from 2mol NaN3 thus 1mol Na from 1 Mol NaN3......
A2 or AS, multi choice or structured papermay june 2003 q 1 and 2 please helppp..urgent....do the working n show me..ty..
paper1 or 2?????may june 2003 q 1 and 2 please helppp..urgent....do the working n show me..ty..
link please....may june 2003 q 1 and 2 please helppp..urgent....do the working n show me..ty..
Well the molecule is a cis-molecule so its either A or C
it has a formula of C20H28O, which is also CnH2n-12O. It has some double bonds, an aldehyde group and a cyclohexene ring
Lets start of with an alkane and change it into the molecule in question
An alkane has a formula of CnH2n+2
If you could change it into an aldehyde, you lose 2hydrogens and gain one Oxygen
so now its CnH2nO
If you could make part of it into a ring, adding a cyclohexane ring, you reduce number of hydrogens by 2. Noticed I added cyclohexane isntead of cyclohexene. We'll take care of double bonds later
now its CnH2n-2O
So now the only group we haven't considered is alkene. Each alkene reduces the H number by 2, so if there are 5 alkenes,
the formula would be CnH2n-12O, which is our molecule
So answer is A
EDIT: Fixed up some mistakes, you should reread it if you read it already
ppr 1paper1 or 2?????
mcqA2 or AS, multi choice or structured paper
which question?Assalamu alaikum,
A friend of mine asked this question. I couldn't solve it
pls explain:
http://www.xtremepapers.com/papers/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_12.pdf
Answer to the question is D.
Apologies. Q.6...which question?
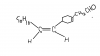
it says that there is an aldehyde group and cyclo hexene so C6H10 + CHO = C7H11Oplease help me with Q20 with explanation.
View attachment 8865
it says that there is an aldehyde group and cyclo hexene so C6H10 + CHO = C7H11O
The real moleculer formula is C20H28O...C20 -C 7= C13....
Now,there iz a long aliphatic chain...general formula is CnH2n+2...so C13H28...
for each double bond formation a hydrogen molecule is removed...so if there iz 5 double bond means ...5*2=10 H is removed...
SO OUT OF 28 H in C13H28...28-10=18 is remained...but 1 more H is removed due to attachment...
no problemApologies. Q.6...
the ion which is reduced gain elecrons.....if we compare chromium then:Apologies. Q.6...
any1 doing chem ppr 12 2morrow? cuz i am & freaking out down here
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now