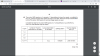
From part b we we know that:
0.04 mol of NaOH reacted with ethanoic acid
mol ratio of NaOH to ethanoic acid in the reaction is 1:1
therefore, there was 0.04 mol of ethanoic acid in the equilibrium state
before the equilibrium state there was 0.1 mol of ethanoic acid
so the other 0.06 mol of ethanoic acid underwent the forward reaction
mole ratio of acid to alcohol is 1:1
0.06 mol of alcohol reacted with the 0.06 mol of acid
this leaves 0.04 mol of alcohol
mole ratio of acid to both products is 1:1
0.06 mol of both products was formed from the 0.06 mol of acid
therefore, table is:
0.04 0.04 0.06 0.06