- Messages
- 25
- Reaction score
- 54
- Points
- 13
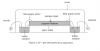
Thank You so much! But once I made a similar diagram and my teacher pointed out that this is close to electrolysis, draw it as it is in marking scheme
Teachers usually treat the mark scheme as the Bible or something lol. No need to draw it exactly as the mark scheme. Just make sure your diagram includes the relevant points quoted in the mark scheme, as those are what was marked :
- power supply
- electrolyte + filter paper
- buffer
- acid mixture central