- Messages
- 155
- Reaction score
- 27
- Points
- 28
what is the answer?
It's A.
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
what is the answer?
Here is what I did, Idont know if there is another way of solving it or not. Anyhow this is how i did it.It's A.
use m*c*delta T using m as your mass of water (because that is what is actually absorbing the heat)PLEASE HELPPPPPPPPPP
Correct answer is B.
I spot 7How do we figure out the number of chiral centres present??
Correct answer is 6.
How do we solve this?!!
Two glass vessels M and N are connected by a closed valve.
M contains helium at 20 °C at a pressure of 1 x 10^5 Pa. N has been evacuated, and has three times the volume of M. In an experiment, the valve is opened and the temperature of the whole apparatus is raised to 100 °C.
What is the final pressure in the system?
a. 3.18 x 10^4 Pa
b. 4.24 x 10^4 Pa
c.1.25 x 10^5 Pa
d.5.09 x 10^5 Pa
Here is what I did, Idont know if there is another way of solving it or not. Anyhow this is how i did it.
First I found the volume of M in terms of n using PV=nRT (there is no way of actually finding the number of moles because they didnt give you the mass of Helium or its volume) Anyhow
Volume of M --> 1x10^5*V=n*8.31*(20+273)
V=0.024n
Now they told you that the volume of N is three times that of M hence Vol of N is 3*V i.e 3*0.024n = 0.073n
Now they asked for the final pressure which means the pressure of both the gasses M and N and they told you that the temp is 100 so what we do is first we find the TOTAL volume of both M and N which is 0.024n+0.073n= 0.097n
so to find the Total pressure just put in your values in the formula
P*0.097n=n*8.31*(100+273) (the n will cancel out)
P=3.18x10^4
BTW when you attempt solving it yourself use the exact values from the calculator, if you use my rounded off values you will get 3.19x10^4
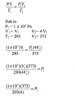
can any kind soul clear my doubts
here are the answers
1.C
7.C
10.A
12.D
14.B
25.C
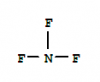
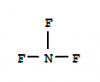

First of all the reaction is hemolytic fission. Then if we draw out the propogation and termination steps of reaction you can see if the chlorine atom joins at first or second carbon atom we get 1-chloropropane whereas we only get 2-chloropopane if it joins at second carbon atom. Hydrogen atoms at 2nd carbon are two and 1st and 3rd carbon are 6. the ratio will be 3:1why is it 1:3 & not 1:1
x = 104.5The answer is C but shouldn't it be A.
why is x 104.5 shouldnt it be 180x = 104.5
y = 109.5
z = 107.5
y -> z -> x
10)can any kind soul clear my doubts
here are the answers
1.C
7.C
10.A
12.D
14.B
25.C
There are two lone pairs. H-O-H isnt 180 coz of lone pair, CO2 is pure sp hybridization without lone pair, hence its linear.why is x 104.5 shouldnt it be 180
PLEASE HELPPPPPPPPPP
Correct answer is B.
oxidant is the one that gets reduced itself right?10)
A) HSO3 is a proton acceptor. Proton acceptor is a base.
B) SO2 is a reductant.
C) 2H+ donated proton to SO3(-2). Hence its base.
D) SO3(-2) is an oxidant.
For more than 16 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now