-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
My Book says :
1.Back Emf seems to imply opposition, something not wanted, yet in a motor it is the induced emf that is responsible for the mechanical work output of the motor.
2.The workdone against the induced emf in a motor is the useful work output of the motor. Workdone against the resistance of the wires is wasted as thermal energy
explain please?
How is back emf responsible for the ouput of a motor? :S isn't motor supposed to provide us force or mechanical energy
1.Back Emf seems to imply opposition, something not wanted, yet in a motor it is the induced emf that is responsible for the mechanical work output of the motor.
2.The workdone against the induced emf in a motor is the useful work output of the motor. Workdone against the resistance of the wires is wasted as thermal energy
explain please?
How is back emf responsible for the ouput of a motor? :S isn't motor supposed to provide us force or mechanical energy
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
the gradient of boyle's law graph represents Temperature or the area under the graph? or neither of them? if we are asked to draw a graph at a higher temp and at a lower temp, why should we draw the graph of higher temp slightly above that for lower temp. I've seen that in several books but fail to understand why
- Messages
- 122
- Reaction score
- 17
- Points
- 28
- Messages
- 1,394
- Reaction score
- 12,123
- Points
- 523
Is the answer B?View attachment 60059
can anyone help me with this qtn...???
- Messages
- 1,394
- Reaction score
- 12,123
- Points
- 523
First consider the left two columns of mercury. The pressure difference between the atmospheric pressure and air inside the colum is given by:View attachment 60059
can anyone help me with this qtn...???
P - 760 = x (we are subtracting 760 from P since we can see that air inside is at a higher pressure than atmospheric pressure)
P = 760 + x ---- (i)
Now consider the right two columns of mercury. The pressure difference b/w the air inside and atmospheric pressure is given by :
P - 760 = 50 - y
P = 810 - y ----(ii)
Solve these two equations (i) & (ii), you'll get,
760 + x = 810 - y
x + y = 50
^ this expression tells us that sum of the values of x & y must be equal to 50, so eliminate all those options which do not sum up to 50. This is will relieve our headache a bit
Now you know that between B & C, there's one culprit option
Substitute the values of x & y from these two options into the equations (i) & (ii). If both the values in an option satisfy both equations, then that will be our required answer.
So put them one by one:
Option B:
780 = 760 +20 (Correct!)
780 = 810 - 30 (Correct!)
Option C:
810 = 760 + 30 (Incorrect!)
810 = 810 - 20 (Again incorrect!)
So the correct option is B.
I hope it makes sense
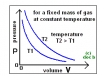
Boyle's Law states that for given mass of gas at a constant temperature (oC or K), the product of the pressure multiplied by the volume is a constantthe gradient of boyle's law graph represents Temperature or the area under the graph? or neither of them? if we are asked to draw a graph at a higher temp and at a lower temp, why should we draw the graph of higher temp slightly above that for lower temp. I've seen that in several books but fail to understand why
The graph shows how the pressure and volume vary according to Boyles Law at two different temperatures
- At lower temperatures the volume and pressure values are lower
- so the gradient of higher temperature T2 is steeper than T1
- Messages
- 25
- Reaction score
- 6
- Points
- 13
http://maxpapers.com/wp-content/uploads/2012/11/9702_s08_qp_4.pdf
please, help me with q5 part b) .
can someone post a picture of the answer( not just the verbal explanation) . because the question asks for the diagram.
thanks in advance.
please, help me with q5 part b) .
can someone post a picture of the answer( not just the verbal explanation) . because the question asks for the diagram.
thanks in advance.
- Messages
- 122
- Reaction score
- 17
- Points
- 28
thanks alots......First consider the left two columns of mercury. The pressure difference between the atmospheric pressure and air inside the colum is given by:
P - 760 = x (we are subtracting 760 from P since we can see that air inside is at a higher pressure than atmospheric pressure)
P = 760 + x ---- (i)
Now consider the right two columns of mercury. The pressure difference b/w the air inside and atmospheric pressure is given by :
P - 760 = 50 - y
P = 810 - y ----(ii)
Solve these two equations (i) & (ii), you'll get,
760 + x = 810 - y
x + y = 50
^ this expression tells us that sum of the values of x & y must be equal to 50, so eliminate all those options which do not sum up to 50. This is will relieve our headache a bitPhew! A & D are finally gone
Now you know that between B & C, there's one culprit option
Substitute the values of x & y from these two options into the equations (i) & (ii). If both the values in an option satisfy both equations, then that will be our required answer.
So put them one by one:
Option B:
780 = 760 +20 (Correct!)
780 = 810 - 30 (Correct!)
Option C:
810 = 760 + 30 (Incorrect!)
810 = 810 - 20 (Again incorrect!)
So the correct option is B.
I hope it makes sense
- Messages
- 122
- Reaction score
- 17
- Points
- 28
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
so basically the temperature is the product of P and V>?View attachment 60060
Boyle's Law states that for given mass of gas at a constant temperature (oC or K), the product of the pressure multiplied by the volume is a constant
The graph shows how the pressure and volume vary according to Boyles Law at two different temperatures
- At lower temperatures the volume and pressure values are lower
- so the gradient of higher temperature T2 is steeper than T1
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
so basically the temperature is the product of P and V>?
actually the product PV is constant at a constant temperature
temperature is not equal to the gradient
it is CONSTANT
as temperature remains constant the same amount of energy given to the system persists throughout its operation and therefore, theoretically, the value of (PV) k will remain constant
however, at a higher constant temperature the product PV will also increase therefore, there is a difference in the gradients
- Messages
- 122
- Reaction score
- 17
- Points
- 28
helppppppppppppp
- Messages
- 2,738
- Reaction score
- 6,309
- Points
- 523
For this use the formula s=ut+1/2at^2
helppppppppppppp
Since the initial velocity is zero so ut can be eliminated and then rearrange to make a the subject which becomes a= 2s/t^2
Now you can see that s and a are directly propotional so an increase in s will result in greater acceleration whereas a and t are inversely propotional. Check all options accordingly. A: If h is smaller, a must be smaller hence incorrect
B: Again h is smaller and t is larger both effects making the a smaller
C: h is larger , yes that increases but then t is also larger which sort of cancels out the effect
D: h is measured correctly while t is small which makes a larger and hence is the correct answer
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Yes that location is fine. I would keep there as well.The question asked us to place a capacitor for smoothing in the circuit
Why should it be across SQ?
eView attachment 60066
- Messages
- 122
- Reaction score
- 17
- Points
- 28
thank uFor this use the formula s=ut+1/2at^2
Since the initial velocity is zero so ut can be eliminated and then rearrange to make a the subject which becomes a= 2s/t^2
Now you can see that s and a are directly propotional so an increase in s will result in greater acceleration whereas a and t are inversely propotional. Check all options accordingly. A: If h is smaller, a must be smaller hence incorrect
B: Again h is smaller and t is larger both effects making the a smaller
C: h is larger , yes that increases but then t is also larger which sort of cancels out the effect
D: h is measured correctly while t is small which makes a larger and hence is the correct answer
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
As energy wont be negative the graph will be always above t - axis.
At mean position, P.E = 0
At maximum displacement, maximum P.E.