-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 122
- Reaction score
- 17
- Points
- 28
- Messages
- 122
- Reaction score
- 17
- Points
- 28
- Messages
- 124
- Reaction score
- 30
- Points
- 38
We use A= -λN because we have Activity, Decay constant and N( the no of particles that are present).View attachment 61806
Can any1 pls xplain (b)?
y do we use A= -λN?
cuz N gives us undecayed nuclei nt amnt in atmosphere?
Using that formula we get N= 9.52 x 10^7 of Radon-222 atoms
So the ratio will be (2.52 ×10^25)/(9.52 x 10^7) giving 2.6 x 10^17. I believe this is the correct answer.
We use A= -λN because we have Activity, Decay constant and N( the no of particles that are present).
Using that formula we get N= 9.52 x 10^7 of Radon-222 atoms
So the ratio will be (2.52 ×10^25)/(9.52 x 10^7) giving 2.6 x 10^17. I believe this is the correct answer.
bt isn't N the no. of particles present in the sample n not in the air? Lyk the undecayed amnt?
- Messages
- 124
- Reaction score
- 30
- Points
- 38
In 1m^3 of atmospheric air 9.52 x 10^7 atoms of Radon-222 are presentbt isn't N the no. of particles present in the sample n not in the air? Lyk the undecayed amnt?
- Messages
- 8,521
- Reaction score
- 34,851
- Points
- 718
I am an Engineering student. Got AAB in Phy, Math, and Chem respectively.Specifically looking for a candidate who appeared in A level exams and received an A and had Physics Chemistry and Biology just wanted few advices regarding how to approach the examintions and how to revise having 40 days in hand
Only forty days remaining. I would strongly encourage you to focus on past papers right now.
Solve as many papers as you can, find in which concepts you are loosing marks.
Target your weaknesses, work on them.
Plan your studies properly. Two papers per subject in a day, if you can manage it. Else, just one paper per subject in a day will be also great.
No one can stop you from an A.
Good luck.
P.S. This is the time. Work on your weaknesses. Strengthen it. A is yours.
- Messages
- 252
- Reaction score
- 123
- Points
- 53
(918 - 900) x 1000 = 18000yes but how?
- Messages
- 1
- Reaction score
- 0
- Points
- 1
- Messages
- 42
- Reaction score
- 6
- Points
- 18
- Messages
- 42
- Reaction score
- 6
- Points
- 18
- Messages
- 42
- Reaction score
- 6
- Points
- 18
- Messages
- 42
- Reaction score
- 6
- Points
- 18
- Messages
- 757
- Reaction score
- 5,221
- Points
- 523
View attachment 61858
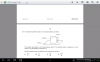
Anweris C .I didnt get how to solve it .please help me.
 Here is the solution to this question. I hope it helps.
Here is the solution to this question. I hope it helps.
Last edited:
- Messages
- 132
- Reaction score
- 74
- Points
- 38
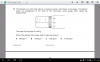
Can anyone explain these questions
View attachment 61855 Anwer is D
Attachments
- Messages
- 132
- Reaction score
- 74
- Points
- 38
View attachment 61856 Answer is C how?
Attachments
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
9702/13 June 2014
Q 12, Q 14, Q 16, Q 34, Q 35, Q 37
Help me out in these Questions please.
Thanks in Advance.
Q 12, Q 14, Q 16, Q 34, Q 35, Q 37
Help me out in these Questions please.
Thanks in Advance.
Last edited: