-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Physics, Chemistry and Biology: Post your doubts here!
- Thread starter semsemhosam
- Start date
- Messages
- 7
- Reaction score
- 0
- Points
- 11
I always find it difficult to solve physics atp... Could you provide me some tips fr help?
- Messages
- 45
- Reaction score
- 31
- Points
- 28
what is the difference between combustion and burning?.
And which one is an exothermic reaction?.
And which one is an exothermic reaction?.
- Messages
- 45
- Reaction score
- 31
- Points
- 28
(i) Fig.10.3 the coil is perpendicular to the magnetic field , so the e.m.f is at its maximum
(ii) Fig.10.1 as the coil is parallel to the magnetic field, thus no e.m.f is produced
- Messages
- 45
- Reaction score
- 31
- Points
- 28
Is neutralisation endothermic or exothermic?
- Messages
- 45
- Reaction score
- 31
- Points
- 28
I am not really sure but I guess we should add their masses (as they will be joined together) and then divide 0.16 (as momentum before the collision is equal to the momentum after the collision) by 0.8 (the sum of their masses) to get the speed(velocity) to be 0.2m/s
- Messages
- 45
- Reaction score
- 31
- Points
- 28
View attachment 65163
View attachment 65164
View attachment 65165
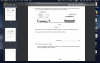
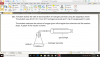
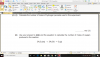
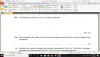
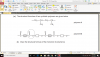
How to do this, please help, I am not understanding this
mole=volume x concentration
volume=mole x 24
4(c)(i) 0.002 (mol); 0.1 x 0.02dm3 (e.g 20cm3/1000)
4(c)(ii) 0.001 (mol); 0.002/2 (becuz its 2H2O2:1O2 SO 2:1)
4(c)(iii) 0.024 (dm3); 0.001 x 24
4(c)(iv) no change/ no effect; (as catalysts don't affect equilibrium and thus the volume of the reactants-it just speeds up the reaction)
4(c)(v) 0.048 (dm3 ); 0.024 x 2 (as concentration is doubled )-e.g 0.1mol/dm3 x 2 = 0.2 mol/dm3
Hope it helps
Good luck!
- Messages
- 45
- Reaction score
- 31
- Points
- 28
Here are some I use. Hope they r beneficialHello everyone, I need some help, can anyone please give me notes for Physics, Chemistry and Biology, thanks
Attachments
thank you very much sir I really appreciated, god bless youHere are some I use. Hope they r beneficial
Thank you very much sir, for helping me again, god bless youmole=volume x concentration
volume=mole x 24
4(c)(i) 0.002 (mol); 0.1 x 0.02dm3 (e.g 20cm3/1000)
4(c)(ii) 0.001 (mol); 0.002/2 (becuz its 2H2O2:1O2 SO 2:1)
4(c)(iii) 0.024 (dm3); 0.001 x 24
4(c)(iv) no change/ no effect; (as catalysts don't affect equilibrium and thus the volume of the reactants-it just speeds up the reaction)
4(c)(v) 0.048 (dm3 ); 0.024 x 2 (as concentration is doubled )-e.g 0.1mol/dm3 x 2 = 0.2 mol/dm3
Hope it helps
Good luck!
- Messages
- 2
- Reaction score
- 0
- Points
- 1